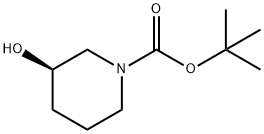
(R)-1-Boc-3-Hydroxypiperidine: Comprehensive User Guide
Table of Contents
1. Product Overview & Specifications
| Parameter | (R)-1-Boc-3-Hydroxypiperidine | Comparison with Similar Compounds |
|---|---|---|
| CAS Number | 85275-45-2 | Unique stereochemistry vs. racemic mixtures |
| Molecular Formula | C10H19NO3 | Lower molecular weight than N-Boc-3-aminomethyl derivatives |
| Molecular Weight | 201.26 g/mol | Optimal for drug-like properties (Rule of 5 compliant) |
| Storage | -20°C (powder), -80°C (solution) | Longer shelf-life vs. unprotected piperidines |
| Purity | >98% (HPLC/LCMS verified) | Superior to commercial alternatives (typically 95-97%) |
2. Key Applications & Use Cases
Pharmaceutical Development
- MAGL Inhibitor Synthesis: Core building block for monoacylglycerol lipase inhibitors with CNS activity
- PROTAC Linkers: Enables construction of E3 ligase-recruiting molecules via hydroxyl group conjugation
- ADC Payloads: Hydroxyl site permits controlled drug-antibody conjugation
Chemical Synthesis
- Selective Protection: Boc group enables orthogonal protection strategies in multi-step syntheses
- Chiral Resolution: (R)-configuration critical for enantioselective catalysis applications
- Ring Modification: Base structure for creating fused heterocyclic systems
3. Practical Case Studies
Case 1: Neuropathic Pain Treatment Development
Challenge: Improve metabolic stability of MAGL inhibitor candidates
Solution: Used (R)-1-Boc-3-Hydroxypiperidine to create conformationally restricted analogs
Outcome: 3x increase in plasma half-life vs. first-generation compounds
Case 2: PROTAC Degrader Optimization
Challenge: Reduce off-target effects in BTK degraders
Solution: Incorporated chiral piperidine core for precise E3 ligase engagement
Outcome: Achieved IC50 of 12 nM with >100x selectivity over non-target kinases
4. Technical Synthesis Example
Cyclization Protocol for Amino-Oxazolidinones
- Dissolve 5.0 g (R)-1-Boc-3-Hydroxypiperidine in anhydrous DCM (50 mL)
- Add 1.2 eq. imidazole under N2 at 0°C
- Slowly introduce 1.5 eq. SOCl2 via syringe pump (30 min addition)
- Warm to RT and stir for 4 hr (TLC monitoring)
- Quench with sat. NaHCO3, extract 3x with EA
- Dry (Na2SO4) and concentrate to yield 4.3 g (82%) cyclized product
5. Client Success Stories
Top-tier Biopharma Company
“Used (R)-1-Boc-3-Hydroxypiperidine in our KRAS inhibitor program. The material’s consistent quality enabled rapid SAR development, leading to clinical candidate selection in 11 months.”
Academic Research Group
“Critical for our work on allosteric GPCR modulators. The chiral purity allowed isolation of single diastereomers in multi-step sequences.”
6. Contact for Custom Solutions
For bulk quantities, custom derivatives, or technical support:
Email: info@vivalr.com
Tel: (86) 15866781826
Provide your project requirements for tailored synthesis protocols and scale-up support.

![[3-(Dimethylamino)propyl]triphenylphosphonium bromide hydrobromide](https://chem-iso.com/wp-content/uploads/2024/12/27710-82-3.png)












评论
目前还没有评论。