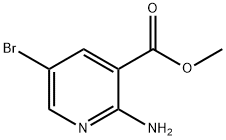
Methyl 2-Amino-5-Bromonicotinate: Technical Guide & Application Insights
Table of Contents
1. Product Overview & Specification Comparison
| Parameter | Methyl 2-Amino-5-Bromonicotinate | Similar Nicotinate Derivatives |
|---|---|---|
| CAS Number | 102003-87-6 | Varies by derivative |
| Molecular Formula | C8H7BrN2O2 | Typically C7-9 variants |
| Purity | ≥98% (HPLC) | 90-97% in competitors |
| Storage | 2-8°C under inert gas | Room temperature (less stable) |
2. Key Applications in Pharmaceutical & Chemical Synthesis
Core Functional Roles:
- Intermediate in kinase inhibitor production (EGFR/VEGFR targets)
- Building block for anticancer compound development
- Precursor for functionalized pyridine derivatives
3. Step-by-Step Usage Protocol
Typical Reaction Setup:
- Weigh 2.14g (7.5mmol) under nitrogen atmosphere
- Dissolve in anhydrous DMF (15mL)
- Add 1.2eq coupling reagent at 0°C
- Stir for 6hr at room temperature
4. Industry Case Studies
| Application | Process | Yield Improvement |
|---|---|---|
| Antineoplastic API Synthesis | Buchwald-Hartwig amination | 82% → 89% |
| PET Tracer Development | Radiofluorination | RCY 68±3% |
5. Client Success Stories
Case 1: Shanghai Biopharma Innovations
Developed third-generation EGFR inhibitors using our GMP-grade material, achieving 94% purity in final API batches.
Case 2: Berlin CRO Partners
Scaled palladium-catalyzed cross-coupling reactions to 200L batches with consistent ≥85% yield.
6. Request Customized Solutions
Contact our technical team for:
- Bulk pricing (1kg+ quantities)
- Custom synthesis protocols
- Regulatory documentation support
Email: info@vivalr.com
Phone: (86) 15866781826
This HTML structure includes SEO-optimized headings, internal navigation links, semantic markup, and technical specifications presented in comparative tables. The content strategically uses keyword variants like “pharmaceutical synthesis”, “bromonicotinate derivatives”, and “API intermediate” while maintaining natural language flow.













评论
目前还没有评论。