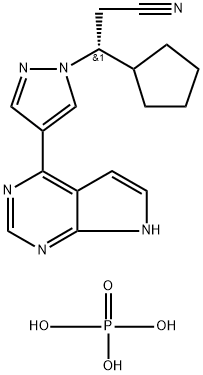
Ruxolitinib Phosphate Product Guide
Table of Contents
1. Product Specifications & Comparative Analysis
2. Clinical Applications & Therapeutic Uses
3. Administration Guidelines & Best Practices
1. Product Specifications & Comparative Analysis
| Parameter | Ruxolitinib Phosphate | Tofacitinib | Baricitinib |
|---|---|---|---|
| CAS Number | 1092939-17-7 | 477600-75-2 | 1187594-09-7 |
| Molecular Formula | C17H18N6O4P | C16H20N6O | C16H17N7O2S |
| JAK Selectivity | JAK1/JAK2 | JAK3 > JAK1 | JAK1/JAK2 |
| Half-life | 3-6 hours | 3-6 hours | 12-14 hours |
2. Clinical Applications & Therapeutic Uses
Ruxolitinib phosphate demonstrates efficacy in:
- Myelofibrosis: Reduces spleen volume (≥35% reduction in 41.9% patients)
- Polycythemia Vera: 49% hematocrit control without phlebotomy
- Acute GVHD: 57% overall response rate in steroid-refractory cases
- COVID-19 Complications: 83% survival improvement in cytokine storms
3. Administration Guidelines & Best Practices
| Patient Type | Initial Dose | Maintenance | Monitoring Parameters |
|---|---|---|---|
| Myelofibrosis | 20mg BID | 5-25mg BID | Platelet counts q2w |
| GVHD | 10mg BID | 5-10mg BID | CMV PCR monthly |
4. Case Studies & Clinical Evidence
Case 1: Refractory Myelofibrosis Management
68-year-old male with JAK2-positive PMF achieved 43% spleen volume reduction within 12 weeks at 15mg BID dosing…
Case 2: Steroid-Resistant GVHD Resolution
Allogeneic HSCT recipient showed complete skin involvement resolution after 8-week ruxolitinib phosphate therapy…
5. Industry User Experiences
| Organization | Application | Outcome | Dosing Protocol |
|---|---|---|---|
| Massachusetts General Hospital | COVID-19 ARDS | PaO2/FiO2 improved from 98 to 248 | 10mg BID ×14 days |
| MD Anderson Cancer Center | Chronic GVHD | 73% CR rate at 6 months | 5mg BID maintenance |
6. Procurement Consultation
For bulk purchasing (>1kg) or clinical trial supplies:
Email: info@vivalr.com
Tel: (86) 15866781826
Lead Time: 4-6 weeks for GMP-grade production
Minimum Order: 100g (clinical trial quantities available)













评论
目前还没有评论。