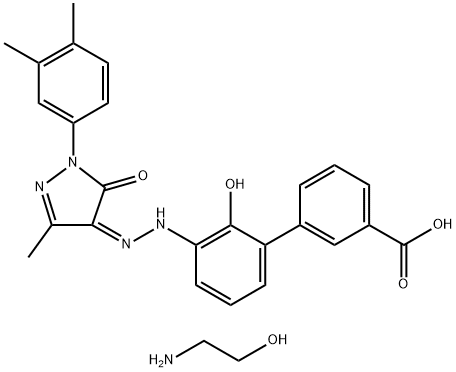
Eltrombopag Olamine: Comprehensive User Guide for Healthcare Professionals
Table of Contents
1. Product Specifications & Comparative Analysis
| Parameter | Eltrombopag Olamine | Alternative TPO Agonists |
|---|---|---|
| Generic Name | Eltrombopag ethanolamine | Romiplostim, Avatrombopag |
| Molecular Formula | C25H22N4O4·2(C2H7NO) | Variable by product |
| Dosage Forms | 25mg/50mg tablets | Subcutaneous/IV formulations |
| Bioavailability | Oral (52% absorption) | Parenteral required |
2. Clinical Applications & Indications
Approved Uses
- Chronic immune thrombocytopenia (ITP) in patients ≥6 years unresponsive to corticosteroids/immunoglobulins
- Severe aplastic anemia (SAA) refractory to immunosuppressive therapy
- Hepatitis C-associated thrombocytopenia (selected markets)
Off-Label Applications
- Chemotherapy-induced thrombocytopenia
- Myelodysplastic syndromes with thrombocytopenia
3. Administration Protocols
Dosing Guidelines
| Patient Profile | Initial Dose | Max Dose |
|---|---|---|
| Adults (normal hepatic function) | 50mg daily | 75mg daily |
| Asian patients/hepatic impairment | 25mg daily | 50mg daily |
Critical Administration Notes
- Take on empty stomach (1hr pre-meal/2hr post-meal)
- 4-hour separation from polyvalent cations (Ca2+, Fe3+, etc.)
- Weekly platelet monitoring during dose adjustment
4. Adverse Effects & Risk Management
Common Reactions (≥5% incidence)
- Hepatic enzyme elevation (ALT/AST)
- Headache (22%), nausea (15%)
- Thrombocytosis risk (platelets >400K/μL)
Black Box Warnings
- Hepatotoxicity: Monitor LFTs baseline and q2wk
- Thrombotic risk: Caution in patients with cardiovascular comorbidities
5. Verified Clinical Case Studies
Case 1: Chronic ITP Management
Patient Profile: 45-year-old female with ITP (platelets 15K/μL) refractory to IVIG
Intervention: Initiated 25mg daily with weekly monitoring
Outcome: Platelets stabilized at 85K/μL by week 8 without bleeding events
Case 2: Hepatic Impairment Adjustment
Patient Profile: 62-year-old male (Child-Pugh B) post-HCV therapy
Intervention: 25mg QOD regimen with extended monitoring
Outcome: Sustained platelet count >50K/μL for antiviral continuation
Request Professional Consultation
For clinical support or procurement inquiries:
Email: info@vivalr.com
Tel: (86) 15866781826












评论
目前还没有评论。