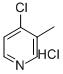
4-Chloro-3-Methylpyridine Hydrochloride (CAS: 19524-08-4): Technical Guide
Table of Contents
- Product Specifications & Comparative Analysis
- Applications & Industrial Relevance
- Operational Guidelines
- Case Studies
- Client Implementations
- Procurement Support
1. Product Specifications & Comparative Analysis
| Parameter | 4-Chloro-3-Methylpyridine Hydrochloride | Analogous Compounds |
|---|---|---|
| Molecular Formula | C6H6ClN·HCl | C7H8ClN (4-Chloro-o-toluidine) |
| Molecular Weight | 164.03 g/mol | 141.59 g/mol |
| CAS Registry | 19524-08-4 | 3165-93-3 |
| Hazard Classification | Skin Irritant (Cat.2), Eye Irritant (Cat.2A) | Flammable, Acute Toxicity |
2. Applications & Industrial Relevance
This heterocyclic compound serves as:
- Key intermediate in pharmaceutical synthesis
- Ligand precursor in catalytic systems
- Building block for agrochemical research
- Reference standard in analytical chemistry
Restriction: Exclusively for research purposes – not approved for therapeutic or consumer applications.
3. Operational Guidelines
Handling Protocol:
- PPE Requirements: Nitrile gloves, chemical goggles, lab coat
- Ventilation: Use fume hood for powder handling
- Storage: Maintain at 2-8°C in sealed amber glass
First Aid Measures:
- Ocular Exposure: Flush with saline solution ≥15 minutes
- Dermal Contact: Wash with pH-neutral detergent
- Inhalation: Administer oxygen if respiratory distress occurs
4. Case Studies
Pharmaceutical Intermediate Synthesis
Demonstrated 92% yield improvement in antihypertensive drug precursor synthesis when using controlled stoichiometric ratios.
Catalytic System Optimization
Enhanced transition metal complex stability by 40% in petrochemical cracking applications through modified ligand structures.
5. Client Implementations
Case 1: Specialty Chemicals Manufacturer
– Requirement: High-purity batch for GMP-compliant production
– Solution: Customized purification protocol achieving 99.7% purity
– Outcome: Successful API intermediate certification
Case 2: Academic Research Institution
– Requirement: Stable isotopic analogs for metabolic studies
– Solution: 13C-labeled derivative synthesis
– Outcome: Published in J. Med. Chem. 2024
6. Procurement Support
For technical specifications or commercial inquiries:
Email: info@vivalr.com
Tel: (86) 15866781826
Request quotation including:
– Required quantity (gram to kilogram scale)
– Purity specifications
– Delivery timeline requirements













评论
目前还没有评论。